Day 1 :
Keynote Forum
Andrew F Christie
University of Melbourne, Australia
Keynote: Follow-on innovation relating to block-buster drugs – innovators, timing, and private value
Time : 11:00-11:30

Biography:
Andrew F Christie was appointed as the inaugural Chair of Intellectual Property at Melbourne Law School, University of Melbourne, in 2002. A former Fulbright Senior Scholar, he has held appointments at the University of Cambridge, Duke University, the National University of Singapore, and the University of Toronto. He is admitted to legal practice in Australia and England, and worked for many years in the intellectual property departments of law firms in Melbourne and London. He is an active Adviser to the Australian Government and to the World Intellectual Property Organization on Intellectual Property Law and Policy. He has been identified by the international magazine Managing IP as one of the world’s 50 most influential people in intellectual property.
Abstract:
Statement of the Problem: The majority of patents associated with high-cost drugs are “secondary patents” – i.e. patents not for the active pharmaceutical ingredient (API), but for follow-on innovation regarding the API, such as means for formulating the API, mechanisms for delivering the API, and methods of medical treatment using the API. Concern has been expressed about the potential for secondary patents to increase the cost of drugs, by extending (“evergreening”) the API originator’s monopoly over the drug. The purpose of this study is to determine whether these concerns are valid.
Methodology & Theoretical Orientation: For a sample of the 13 most costly drugs in Australia over a decade, we identify all the secondary patents granted in Australia for that drug (of which there are 636). We analyze those patents to determine by whom and when they were sought, for what type of innovation they were granted, and for how long they lasted.
Findings: Nearly three-quarters of all the secondary patents are owned by an entity other than the originator of the API of the drug. The patents were sought throughout the life of the original patent on the drug’s API. Patents for delivery mechanisms or formulations of the API have a greater private value than patents for other types of innovations relating to the API.
Conclusion & Significance: The vast majority of follow-on innovation over blockbuster drugs is undertaken by competitors of the drug’s originator, and occurs well before (in addition to well after) the expiry of the API patent. This is of significance to policy-makers, as it suggests that any concern that the grant of the API patent over a blockbuster drug will preclude follow-on innovation by competitors is not well founded.
Keynote Forum
Jennifer A Martin
Air Force Research Laboratories, USA
Keynote: Sweat sample processing, method development, and bioinformatic analysis for biomarkers of physical fatigue
Time : xxxx

Biography:
Abstract:
Keynote Forum
Roger M. Leblanc
University of Miami, USA
Keynote: Development and bioapplications of nontoxic carbon dots
Time : 13:40-14:00

Biography:
Abstract:
Keynote Forum
Tamas Sohajda
Cyclolab Ltd., Hungary
Keynote: Cyclodextrin-based formulations: The present and the future
Time : 14:00-14:20

Biography:
Abstract:
Keynote Forum
Samuel Kyeremateng
AbbVie Deutschland GmbH & Co. KG, Germany
Keynote: Optimizing early phase development of amorphous solid dispersion formulation thorough application of modeling tools
Time : 14:20-14:40

Biography:
Abstract:
Keynote Forum
Supamas Napavichayanun
Chulalongkorn University, Thailand
Keynote: Properties of bacterial cellulose wound dressing containing sericin and polyhexamethylene biguanide
Time : sdf

Biography:
Abstract:
- Pharmaceutical Formulations | Liquid Dosage Forms | Pharmaceutical Analysis
Location: Thon Hotel Bristol Stephanie

Chair
Roger M. Leblanc
University of Miami, USA
Session Introduction
Roger M Leblanc
University of Miami, USA
Title: Development and bioapplications of nontoxic carbon dots

Biography:
Roger M Leblanc received his BS in Chemistry in 1964 from Universite Laval, Canada, and PhD in Physical Chemistry in l968 from the same university. From 1968 to 1970, he was a Postdoctoral Fellow in the Laboratory of Prof. George Porter, FRS, in Davy Faraday Research Lab, the Royal Institution of Great Britain. He was a Professor from 1970 to 1993 at Department of Chemistry and Biology in Universite du Quebec a Trois Rivieres, Canada. During this period, he was Chair from 1971 to 1975 at the same department, and Director from 1981 to 1991 at Photobiophysics Research Center. In 1994, he moved to University of Miami, where he has been a Professor at Department of Chemistry since then to present. At University of Miami, he was Chair of Department of Chemistry from 1994 to 2002, and he is appointed as Chair from 2013 to present. He was also one of the three editors of Colloids and Surfaces B: Biointerfaces from 1998 to 2017. During his early career as a Scientist, his research interest was on the photosynthesis and photoconductivity using surface chemistry and spectroscopy. His current research interest is to apply 2-dimensional (2-D) surface chemistry combined with spectroscopy and microscopy to investigate the properties of nanomaterials (carbon dots, graphene oxide and quantum dots) and the fibrillation process of amyloidogenic proteins (insulin, amyloid-beta peptide and islet amyloid polypeptide). He is also interested to design and develop biosensors with high sensitivity and selectivity for diseases diagnosis. He has published 512 scientific articles in peer-reviewed journals. As a Professor, he has supervised more than 100 Master’s and PhD students.
Abstract:
Carbon dots (C-Dots) with diameter smaller than 10 nm have recently attracted enormous attention in various fields due to their unique properties. In this talk, the synthesis, characterization and bioapplications of a new type of nontoxic, water-soluble C-Dots will be presented. A major medical challenge one faces to treat central nervous system (CNS) related diseases is to cross the tight junctions between endothelial cells, which are known as blood-brain barrier (BBB). Recently, our in vivo experimental observations suggested that the transferrin conjugated C-Dots could enter the CNS of Zebrafish while C-Dots alone could not. Thanks to the abundant presence of carboxylic acids on the surface, C Dots are easily conjugated with transferrin and anticancer drug doxorubicin. The system was then applied as a drug delivery system for the delivery of doxorubicin into cancerous cells. Our in vitro study showed greater uptake of the conjugates compared to free doxorubicin, the conjugates at 10 nM was significantly more cytotoxic than doxorubicin alone, reducing viability by 14-45 %, across multiple pediatric brain tumor cell lines. Accidents, disease and aging compromise the structural and physiological functions of bones, and in vivo bone imaging test is critical to identify, detect and diagnose bone related development and dysfunctions. Here we show that C-Dots with low quantum yield ("dark") bind to calcified bone structures of live Zebrafish larvae with high affinity and selectivity. Binding resulted in a strong enhancement of luminescence that was not observed in other tissues, including non-calcified endochondral elements. Retention of C dots by bones was very stable, long lasting, and with no detectable toxicity. These observations support a novel and revolutionary use of C-Dots as highly specific bioagents for bone imaging and diagnosis, and as a potential bone-specific drug delivery carrier.
Tamas Sohajda
Cyclolab Ltd, Hungary
Title: Cyclodextrin-based formulations: the present and the future

Biography:
Tamás Sohajda has been working at CycloLab Ltd., for six years. Currently he is the Director of Research and Development. After graduating as a Pharmacist, he obtained degrees in Pharmaceutical Economy and Quality Assurance. He wrote his PhD thesis on the investigation and understanding of cyclodextrin complexes on a molecular level studying a great number of biological activities and CD derivatives. At CycloLab Ltd. he has been coordinating all development and research works aimed at various and diverse fields such as developing new, cyclodextrin-aided formulations (solid and semi-solid dosage forms, parenterals, etc.), preparing generic formulations or improvements of current products by introducing CDs, designing new industrially important CD derivatives ideal as next generation excipients, drug delivery systems or as biologically active compounds.
Abstract:
Cyclodextrins (CDs) are “cone-shaped” cyclic oligosaccharides, with a hydrophobic cavity and hydrophilic outer surface. These nanoscale substances have long been used as functional excipients in different pharmaceuticals due to their ability to encapsulate drugs and alter their disadvantageous features, e.g. increase the aqueous solubility, improve bioavailability, enhance chemical stability or simply to mask their taste. At present more than 40, CD-enabled human pharmaceutical products are on the market. The present of CDs is thus dominantly being used as excipients. The most favored are 2-hydroxypropyl-β-CD (HPBCD) and sulfobutylether-β-CD, applied in dozens of parenteral formulations, while other derivatives are used for oral, topical, nasal or ocular administration. The marvel of CDs lies in their flexibility: you can optimize the excipient for the active ingredient and for the purpose simultaneously. In the present lecture illustrative drug products will be highlighted demonstrating the applicability of derivatized oligosaccharides in the development of drug formulations. Outlook to the future potential related to drug delivery use of CDs will also be provided. CDs tagged with biological recognition-based labeling were prepared in order to deliver specific drugs to the site of action. Also, combinations with the application of colloidal structures (dispersions, liquid crystals and macromolecules) will be discussed. Yet the future of CDs is not limited to being used as excipients. We can harvest from their complex forming ability in vivo as well, in products, containing CDs as APIs. While the initial idea was to use CDs as detoxification agents or to selectively remove chemicals from the system (e.g. Sugammadex – Bridion), this concept has grown into clinical trials and studies using CDs as API alone (HPBCD as an orphan drug against Niemann-Pick C, Focal Segmental Glomerulosclerosis or Alport Syndrome). A number of further therapeutic applications of CDs themselves are expected to come, some potential areas will be reviewed.
Samuel Kyeremateng
AbbVie Deutschland GmbH & Co. KG, Germany
Title: Optimizing early phase development of amorphous solid dispersion formulation thorough application of modeling tools

Biography:
Samuel Kyeremateng is a Senior Scientist in the Global Pharmaceutical Sciences Division at AbbVie Deutschland in Ludwigshafen. His research activities focus on scientific advances in the understanding of amorphous molecular solids, and development and application of models in predicting with confidence the preferred composition, manufacturing process, and stability of amorphous solid dispersion formulations. His current responsibilities at AbbVie Deutschland include leading the Material Science Group that supports formulation development, and mentoring Doctorate research students and other scientists within the company. He received his Doctorate in Polymer Science from Martin-Luther-Universität in Germany.
Abstract:
Statement of the Problem: Amorphous solid dispersion (ASD) is an established formulation technique for improving the bioavailability of poorly water-soluble active pharmaceutical ingredients (APIs) by increasing solubility, wettability and dissolution rate. Successful manufacturing of ASD formulation by hot melt extrusion (HME) requires selection of e.g. the right API load, excipients, and processing temperature. API load is also crucial in determining important quality attributes of the drug product such as long term physical stability to ensure consistent product performance during its self-life. Identifying the possible maximum drug load limit and excipients for HME feasibility and risk assessment, and long-term physical stability of the manufactured ASD can be quite challenging whereby several extrusion trials are required in addition to prolonged stability studies. Exploring the optimal design space during early phase of formulation development by this approach requires significant amount of resources including API which may be limitedly available during this phase.
Methodology & Theoretical Orientation: As an API-sparing approach, novel empirical model and the rigorous thermodynamic Perturbed Chain Statistically Associating Fluid Theory (PC-SAFT) were applied to model ASD phase diagram of several formulations to effectively and quickly explore the design space to optimize formulation development. These were followed up with HME manufacturing and long-term stability studies (up to 18 months) of the formulations under ICH conditions to verify the model-predicted results. Several APIs and polymeric excipients including Soluplus, Copovidone, PVP, and HPMCAS were used in the studies.
Findings: The modeling tools were found to be very suitable in estimating extrusion temperature required for generating crystal-free ASD formulations as well as predicting their physical stability under different storage conditions, i.e., temperature and relative humidity.
Conclusion & Significance: Recent advances in predictive ASD phase diagram modeling proved to be reliable tools for excipient selection, HME temperature prediction, and designing ASD formulations for maximum drug load and physical stability. Applying these tools enables successful ASD formulation optimization using less resources and materials.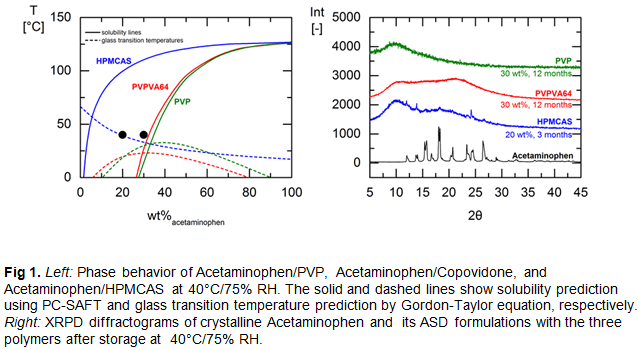
Supamas Napavichayanun
Chulalongkorn University, Thailand
Title: Properties of bacterial cellulose wound dressing containing sericin and polyhexamethylene biguanide

Biography:
Supamas Napavichayanun is a PhD student at the Faculty of Pharmaceutical Sciences, Chulalongkorn University, Thailand. She earned a BSc from Faculty of Pharmaceutical Sciences, Chulalongkorn University in 2010. Her research experience has ranged from protein including silk proteins and biomaterials. She also did clinical researches in the area of dermatology especially materials for wound healing application.
Abstract:
The ideal wound dressing should be a moist and oxygen-permeated environment, exudate adsorption, enhanced wound closure, and infection protection. The bacterial cellulose wound dressing containing sericin and polyhexamethylene biguanide (PHMB) is a natural wound dressing that is easily produced from bacterial cellulose (A. xylinum strain in coconut water medium), silk sericin (protein from silk cocoon), and antiseptic (PHMB). Components of this dressing contain many benefits closely to the ideal wound dressing properties. For the dressing production, the bacterial cellulose dressing was loaded with 1% w/v silk sericin followed by 0.3% w/v PHMB loading. All processes were carried out in sterile conditions. After preparation, the dressings were sterilized with gamma radiation at 25 kGy. The properties of the dressing were tested in term of sericin and antimicrobial releasing, antimicrobial property, and collagen type I production test comparing with commercial product. The results showed that the sufficient concentration for elimination of all bacteria (S. aureus, MRSA, B. subtilis, E. coli, P.aeruginosa, A. baumannii) of PHMB was released from the dressing within 30 minutes and optimal concentration for collagen type I production of sericin was released within 4 hours. The dressing was superior in terms of antimicrobial activity against all bacterial strains than Bactigras®. In comparison with silver-loaded Acticoat®, the antimicrobial activity of the dressing was better against Gram-positive bacteria often found in chronic wounds (S. aureus and MRSA). The antimicrobial difference between the dressing and Suprasorb®X + PHMB was only noticed for B. subtilis. Moreover, the cells cultured from the released solution of our novel dressing produced significantly higher amount of collagen type 1 than those cultured with the bacterial cellulose wound dressing without silk sericin. Therefore, the bacterial cellulose wound dressing containing sericin and PHMB contains many advantages to be the ideal wound dressing.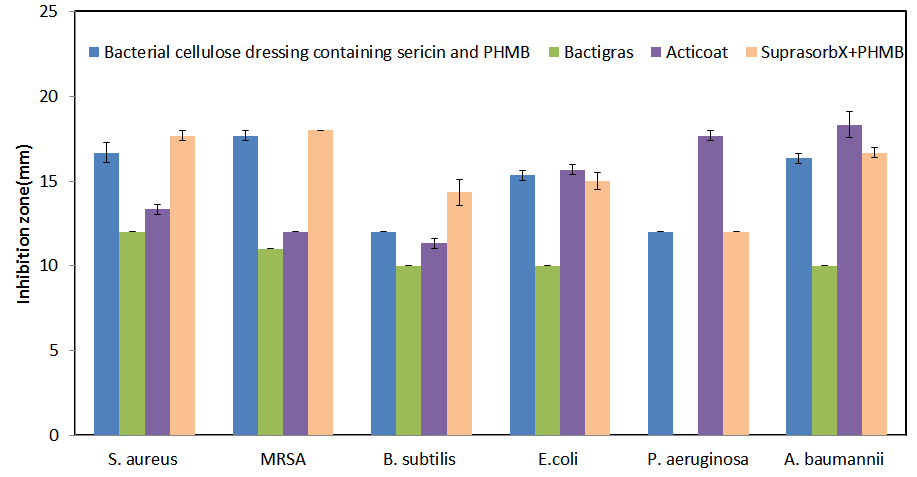
- Pharmaceutical Formulations | Solid Dosage Forms | Novel Drug Delivery Systems | Drug Formulation Procedures | Pharmaceutical Excipients | Regulatory Affairs
Location: Thon Hotel Bristol Stephanie

Chair
Nagatoshi Nishiwaki
Kochi University, Japan
Session Introduction
Nagatoshi Nishiwaki
Kochi University of Technology, Japan
Title: Synthesis of versatile aza-heterocyclic compounds by three component ring transformation

Biography:
Nagatoshi Nishiwaki received his PhD from Osaka University in 1991. He worked at Osaka Kyoiku University (1991–2008). From 2000 to 2001, he joined Karl Anker Jørgensen’s group at Aarhus University, Denmark. Between 2008 and 2009, he worked at Anan National College of Technology. He then moved to the School of Environmental Science and Engineering, Kochi University of Technology, in 2009, and he became a Professor in 2011. His research interests comprise synthetic organic chemistry using nitro compounds, heterocycles (ring transformations, 1,3-dipolar cycloadditions etc.), and pseudo- intramolecular reactions. He has more than 120 papers and 20 review articles.
Abstract:
Dinitropyridone 1 is an excellent substrate for the nucleophilic type ring transformation to afford heterocyclic compounds and nitroanilines those are not easily available by alternative methods. When pyridone 1 was reacted with aromatic ketone in the presence of NH4OAc, 6-arylated 3-nitropyriines 2 were formed besides bicyclic compounds 3. This method was also applicable to synthesis of cycloalka[b]pyridines 4 and 6-alkynylated/alkenylated pyridines 5, respectively. It was found to be possible to use aldehydes as the substrate, which leading to 3,5-disubstituted pyridines 6. On the other hand, when aliphatic ketones were employed as the substrate, two kinds of ring transformation proceeded. Namely, 2,6-disubstituted 4-nitroanilines 8 were formed in addition to nitropyridines 7. It was successful to apply this protocol to synthesis of N,N,2,6-tetrasubstituted nitroanilines 9 upon treatment of dinitropyridone 1 with ketone and amine in the presence of acetic acid.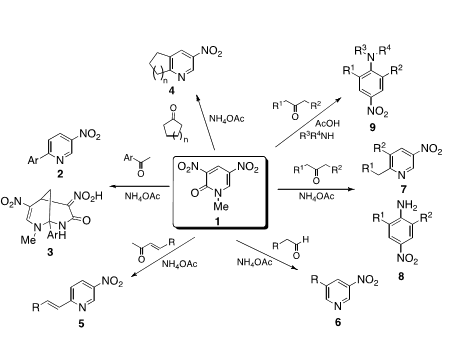
Christian Luebbert
TU Dortmund University, Germany
Title: Long-term stability of pharmaceutical formulations - prediction of recrystallization and amorphous-amorphous phase separation

Biography:
Christian Luebbert graduated in Chemical Engineering at TU Dortmund University, Germany in 2014. During his work as Research Assistant at the Laboratory of Thermodynamics, he focuses on the physical long-term stability of amorphous pharmaceutical formulations. With his expertise, he contributes from an engineering point of view to pharmaceutically highly relevant development of formulation strategies for poorly water-soluble drugs.
Abstract:
Numerous recently-developed active pharmaceutical ingredients (APIs) have a low solubility in water leading to insufficient absorption and bioavailability. To overcome this solubility limitation, APIs are molecularly dispersed in hydrophilic polymers. The resulting formulations are denoted as amorphous solid dispersion (ASDs). For the administration of new pharmaceutical formulations, long-term stability tests are imposed by regulatory authorities at defined conditions of temperature and humidity (25°C, 60% relative humidity (RH) for 12 months tests and 40°C, 75% RH for accelerated six-months tests). Recrystallization of the amorphous API and/or moisture-induced amorphous-amorphous phase separation (miAPS) might occur during storage indicating the thermodynamic instability of the ASDs. Long-term stable formulations are nowadays identified by trial-and-error principles. The aim of this work was to a-priory estimate the long-term stability of ASDs by applying advanced thermodynamic methods1 and thus to reduce the experimental effort for finding promising polymeric carriers suitable for formulation development. In order to validate the thermodynamic predictions, ASDs with different API/polymer compositions were prepared and subjected to two years enduring long-term stability tests at the aforementioned conditions. Recurring PXRD measurements were performed to detect recrystallization and Raman mapping was applied to quantify miAPS. Water sorption was observed as function of time using a magnetic suspension balance. Water sorption and thereby induced phase transitions (recrystallization/ miAPS) could be predicted in quantitative agreement with the experimental data. This study showed that results of long-term stability tests can be predicted correctly in early stages of drug development and that promising polymer candidates for long-term stable ASDs can be identified prior to long-term stability tests by thermodynamic modeling.
Shrawan Baghel
Waterford Institute of Technology, Ireland
Title: Investigating the dissolution performance of dipyridamole and cinnarizine spray dried amorphous solid dispersion using proton NMR

Biography:
Shrawan Baghel is currently doing PhD in “Novel technologies and optimized formulations for delivery of solid dispersion of BCS class II drugs” at Pharmaceutical and Molecular Biotechnology Research Center (PMBRC), Waterford Institute of Technology. He is the winner of Science Foundation Ireland scholarship for this project in collaboration with Synthesis and Solid State Pharmaceutical Centre. The main aim of this project is to gain an insight into the mechanistic and molecular aspects of solid dispersion prepared by spray drying, hot melt extrusion and supercritical fluid process using DSC, XRD and NMR. He had also planned, conducted, interpreted nanotechnology and lipid based formulation approaches to increase the solubility and dissolution of poorly soluble drugs.
Abstract:
Amorphous solid dispersions (ASDs) are of great interest as enabling formulations because of their ability to increase the bioavailability of poorly soluble drugs. However, the dissolution of these ASD based formulations results in highly supersaturated drug solution that can undergo different types of phase transition. We have investigated the dissolution performance of amorphous solid dispersions of poorly water-soluble dipyridamole (DPM) and cinnarizine (CNZ) spray-dried amorphous solid dispersions (ASDs) using polyvinyl pyrrolidone (PVP) and polyacrylic acid (PAA) as a carrier matrix. Dissolution studies were carried out under non sink conditions and solution phase drug-polymer interactions was characterized using proton NMR. It was found that the dissolution of ASDs led to sustained supersaturation, the duration of which varied depending on the drug loading and type of polymer used in the formulation. The main mechanism for drug supersaturation generation and prolongation was found to be anti-plasticization effect of polymers on amorphous drugs within spray dried ASDs and the ability of polymers to reduce the crystal growth rates of DPM and CNZ. To further understand the molecular mechanism behind supersaturation stabilization in the presence of polymer, we employed, Solution 1H NMR. The change in electron densities of proton and the relative intensities of peak shifts indicated the nature of interaction between drug and polymer in different systems are different. These different effects suggest that DPM and CNZ interacts in a different way with PVP and PAA in solution which goes some way towards explaining the different polymeric effect, particularly in terms of inhibition of drug recrystallization and dissolution of DPM and CNZ ASDs. . The overall supersaturation profile observed thus depended on a complex interplay between dissolution rate, polymer type, drug loading, crystallization mechanism of drugs and drug-polymer interaction in the solution state.

Biography:
Shahnaz Usman has received her PhD in Pharmaceutics from University of Karachi during the period of 2007. Currently, she is working as an Associate Professor in Department of Pharmaceutics in RAK College of Pharmaceutical Sciences. She is appointed as Reviewer by different international journals. She is a registered member of Pakistan Pharmacist Association.
Abstract:
Objective: The aim of the present study was to prepare ODT by using relatively a simple direct compression technique with high mechanical strength while keeping the attributes of fast disintegration and dissolution to improve the bioavailability of the drug.
Method: Mannitol and microcrystalline cellulose were studied as diluents in the same quantity for manufacture of montelukast sodium tablet using crospovidone as super disintegrants and sodium bicarbonate as wicking agent. The blend was examined for angle of repose, bulk and tapped density, compressibility index, and Hausner’s ratio. The drug-excipients interaction was investigated by FTIR. After compression hardness, friability, disintegration and dissolution, all the formulations batches were analyzed.
Results: It was found that microcrystalline cellulose was suitable diluent for tablets considering hardness, friability and disintegration time. Ten formulations F1 to F10 were prepared by central composite methods (two level factorial designs) for the selection of optimum concentration of disintegrants and diluents.
Conclusions: The overall results showed that crospovidone was the best super-disintegrant for showing the shortest disintegration time while MCC was the good diluent in preparing montelukast Oro-dispersible tablet and this suggested the possibility of utilizing the selected best formula (F2) in the preparation of Oro-dispersible tablet as a new dosage form for oral administration.

