Day 2 :
Keynote Forum
Gabriele Sadowski
TU Dortmund University, Germany
Keynote: Physical stability and drug crystallization kinetics in amorphous solid dispersions
Time : 11:15-11:45

Biography:
Abstract:
Keynote Forum
Nagatoshi Nishiwaki
Kochi University, Japan
Keynote: Synthesis of versatile aza-heterocyclic compounds by three component ring transformation
Time : dgg

Biography:
Abstract:
Keynote Forum
Christian Luebbert
TU Dortmund University, Germany
Keynote: Long-term stability of pharmaceutical formulations - prediction of recrystallization and amorphousamorphous phase separation
Time : fghhh

Biography:
Abstract:
Keynote Forum
Shrawan Baghel
Waterford Institute of Technology, Ireland
Keynote: Investigating the dissolution performance of dipyridamole and cinnarizine spray dried amorphous solid dispersion using proton NMR
Time : 14:45-15:05

Biography:
Abstract:
Keynote Forum
Shahnaz Usman
RAK MHSU, UAE
Keynote: Formulation and in-vitro evaluation of montelukast oral disintegrating (5 mg) tablets: Effects of diluents
Time : affffffffff

Biography:
Abstract:
Keynote Forum
Tomasz Urbaniak
Wroclaw Medical University, Poland
Keynote: Lamivudine-poly-E-caprolactone conjugate based particles for targeted drug delivery, synthesis and characterization
Time : 15:55 - 16:30

Biography:
Abstract:
Keynote Forum
Supamas Napavichayanun
Chulalongkorn University, Thailand
Keynote: Tolerogenicity, anti-melanogenicity and anti-tyrosinase effects of sericin on melanocyte and dendritic cells: A possibility to alleviate post inflammatory hyperpigmentation
Time : 15:55 - 16:30

Biography:
Abstract:
Keynote Forum
Kampanart Huanbutta
Burapha University, Thailand
Keynote: Development and characterization of seed gums from Cassia fistula as disintegrating agent for fast disintegrating Thai cordial tablet
Time : dff

Biography:
Abstract:
- Pharmaceutical Formulations | Solid Dosage Forms | Novel Drug Delivery Systems | Drug Formulation Procedures | Pharmaceutical Excipients | Regulatory Affairs
Location: Thon Hotel Bristol Stephanie

Chair
Nagatoshi Nishiwaki
Kochi University, Japan
Session Introduction
Nagatoshi Nishiwaki
Kochi University of Technology, Japan
Title: Synthesis of versatile aza-heterocyclic compounds by three component ring transformation

Biography:
Nagatoshi Nishiwaki received his PhD from Osaka University in 1991. He worked at Osaka Kyoiku University (1991–2008). From 2000 to 2001, he joined Karl Anker Jørgensen’s group at Aarhus University, Denmark. Between 2008 and 2009, he worked at Anan National College of Technology. He then moved to the School of Environmental Science and Engineering, Kochi University of Technology, in 2009, and he became a Professor in 2011. His research interests comprise synthetic organic chemistry using nitro compounds, heterocycles (ring transformations, 1,3-dipolar cycloadditions etc.), and pseudo- intramolecular reactions. He has more than 120 papers and 20 review articles.
Abstract:
Dinitropyridone 1 is an excellent substrate for the nucleophilic type ring transformation to afford heterocyclic compounds and nitroanilines those are not easily available by alternative methods. When pyridone 1 was reacted with aromatic ketone in the presence of NH4OAc, 6-arylated 3-nitropyriines 2 were formed besides bicyclic compounds 3. This method was also applicable to synthesis of cycloalka[b]pyridines 4 and 6-alkynylated/alkenylated pyridines 5, respectively. It was found to be possible to use aldehydes as the substrate, which leading to 3,5-disubstituted pyridines 6. On the other hand, when aliphatic ketones were employed as the substrate, two kinds of ring transformation proceeded. Namely, 2,6-disubstituted 4-nitroanilines 8 were formed in addition to nitropyridines 7. It was successful to apply this protocol to synthesis of N,N,2,6-tetrasubstituted nitroanilines 9 upon treatment of dinitropyridone 1 with ketone and amine in the presence of acetic acid.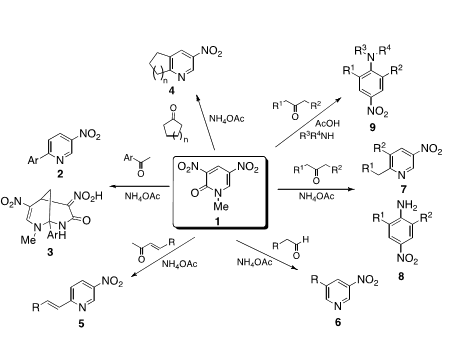
Christian Luebbert
TU Dortmund University, Germany
Title: Long-term stability of pharmaceutical formulations - prediction of recrystallization and amorphous-amorphous phase separation

Biography:
Christian Luebbert graduated in Chemical Engineering at TU Dortmund University, Germany in 2014. During his work as Research Assistant at the Laboratory of Thermodynamics, he focuses on the physical long-term stability of amorphous pharmaceutical formulations. With his expertise, he contributes from an engineering point of view to pharmaceutically highly relevant development of formulation strategies for poorly water-soluble drugs.
Abstract:
Numerous recently-developed active pharmaceutical ingredients (APIs) have a low solubility in water leading to insufficient absorption and bioavailability. To overcome this solubility limitation, APIs are molecularly dispersed in hydrophilic polymers. The resulting formulations are denoted as amorphous solid dispersion (ASDs). For the administration of new pharmaceutical formulations, long-term stability tests are imposed by regulatory authorities at defined conditions of temperature and humidity (25°C, 60% relative humidity (RH) for 12 months tests and 40°C, 75% RH for accelerated six-months tests). Recrystallization of the amorphous API and/or moisture-induced amorphous-amorphous phase separation (miAPS) might occur during storage indicating the thermodynamic instability of the ASDs. Long-term stable formulations are nowadays identified by trial-and-error principles. The aim of this work was to a-priory estimate the long-term stability of ASDs by applying advanced thermodynamic methods1 and thus to reduce the experimental effort for finding promising polymeric carriers suitable for formulation development. In order to validate the thermodynamic predictions, ASDs with different API/polymer compositions were prepared and subjected to two years enduring long-term stability tests at the aforementioned conditions. Recurring PXRD measurements were performed to detect recrystallization and Raman mapping was applied to quantify miAPS. Water sorption was observed as function of time using a magnetic suspension balance. Water sorption and thereby induced phase transitions (recrystallization/ miAPS) could be predicted in quantitative agreement with the experimental data. This study showed that results of long-term stability tests can be predicted correctly in early stages of drug development and that promising polymer candidates for long-term stable ASDs can be identified prior to long-term stability tests by thermodynamic modeling.
Shrawan Baghel
Waterford Institute of Technology, Ireland
Title: Investigating the dissolution performance of dipyridamole and cinnarizine spray dried amorphous solid dispersion using proton NMR

Biography:
Shrawan Baghel is currently doing PhD in “Novel technologies and optimized formulations for delivery of solid dispersion of BCS class II drugs” at Pharmaceutical and Molecular Biotechnology Research Center (PMBRC), Waterford Institute of Technology. He is the winner of Science Foundation Ireland scholarship for this project in collaboration with Synthesis and Solid State Pharmaceutical Centre. The main aim of this project is to gain an insight into the mechanistic and molecular aspects of solid dispersion prepared by spray drying, hot melt extrusion and supercritical fluid process using DSC, XRD and NMR. He had also planned, conducted, interpreted nanotechnology and lipid based formulation approaches to increase the solubility and dissolution of poorly soluble drugs.
Abstract:
Amorphous solid dispersions (ASDs) are of great interest as enabling formulations because of their ability to increase the bioavailability of poorly soluble drugs. However, the dissolution of these ASD based formulations results in highly supersaturated drug solution that can undergo different types of phase transition. We have investigated the dissolution performance of amorphous solid dispersions of poorly water-soluble dipyridamole (DPM) and cinnarizine (CNZ) spray-dried amorphous solid dispersions (ASDs) using polyvinyl pyrrolidone (PVP) and polyacrylic acid (PAA) as a carrier matrix. Dissolution studies were carried out under non sink conditions and solution phase drug-polymer interactions was characterized using proton NMR. It was found that the dissolution of ASDs led to sustained supersaturation, the duration of which varied depending on the drug loading and type of polymer used in the formulation. The main mechanism for drug supersaturation generation and prolongation was found to be anti-plasticization effect of polymers on amorphous drugs within spray dried ASDs and the ability of polymers to reduce the crystal growth rates of DPM and CNZ. To further understand the molecular mechanism behind supersaturation stabilization in the presence of polymer, we employed, Solution 1H NMR. The change in electron densities of proton and the relative intensities of peak shifts indicated the nature of interaction between drug and polymer in different systems are different. These different effects suggest that DPM and CNZ interacts in a different way with PVP and PAA in solution which goes some way towards explaining the different polymeric effect, particularly in terms of inhibition of drug recrystallization and dissolution of DPM and CNZ ASDs. . The overall supersaturation profile observed thus depended on a complex interplay between dissolution rate, polymer type, drug loading, crystallization mechanism of drugs and drug-polymer interaction in the solution state.

Biography:
Shahnaz Usman has received her PhD in Pharmaceutics from University of Karachi during the period of 2007. Currently, she is working as an Associate Professor in Department of Pharmaceutics in RAK College of Pharmaceutical Sciences. She is appointed as Reviewer by different international journals. She is a registered member of Pakistan Pharmacist Association.
Abstract:
Objective: The aim of the present study was to prepare ODT by using relatively a simple direct compression technique with high mechanical strength while keeping the attributes of fast disintegration and dissolution to improve the bioavailability of the drug.
Method: Mannitol and microcrystalline cellulose were studied as diluents in the same quantity for manufacture of montelukast sodium tablet using crospovidone as super disintegrants and sodium bicarbonate as wicking agent. The blend was examined for angle of repose, bulk and tapped density, compressibility index, and Hausner’s ratio. The drug-excipients interaction was investigated by FTIR. After compression hardness, friability, disintegration and dissolution, all the formulations batches were analyzed.
Results: It was found that microcrystalline cellulose was suitable diluent for tablets considering hardness, friability and disintegration time. Ten formulations F1 to F10 were prepared by central composite methods (two level factorial designs) for the selection of optimum concentration of disintegrants and diluents.
Conclusions: The overall results showed that crospovidone was the best super-disintegrant for showing the shortest disintegration time while MCC was the good diluent in preparing montelukast Oro-dispersible tablet and this suggested the possibility of utilizing the selected best formula (F2) in the preparation of Oro-dispersible tablet as a new dosage form for oral administration.
